







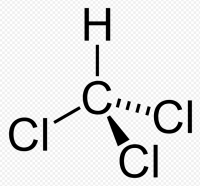
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes.The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered hazardous. Several million tons are produced annually as a precursor to PTFE and refrigerants, but its use for refrigerants is being phased out.The hydrogen attached to carbon in chloroform participates in hydrogen bonding.
History
Trichloromethane was synthesized independently by two groups in 1831: Liebig carried out the alkaline cleavage of chloral, whereas Soubeirain obtained the compound by the action of chlorine bleach on both ethanol and acetone. In 1835, Dumas prepared the substance by the alkaline cleavage of trichloroacetic acid. Regnault prepared trichloromethane by chlorination of monochloromethane. By the 1850s, chloroform was being produced on a commercial basis by using the Liebig procedure, which retained its importance until the 1960s. Today, trichloromethane — along with dichloromethane — is prepared exclusively and on a massive scale by the chlorination of methane and monochloromethane.