







Total internal reflection fluorescence microscopy (TIRFM) is an elegant optical technique utilized to observe single molecule fluorescence at surfaces and interfaces. The technique is commonly employed to investigate the interaction of molecules with surfaces, an area which is of fundamental importance to a wide spectrum of disciplines in cell and molecular biology.
History
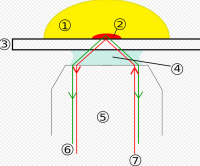
The idea of using total internal reflection to illuminate cells contacting the surface of glass was first described by E.J. Ambrose in 1956.This idea was then extended by Daniel Axelrod at the University of Michigan, Ann Arbor in the early 1980s as TIRFM. A TIRFM uses an evanescent wave to selectively illuminate and excite fluorophores in a restricted region of the specimen immediately adjacent to the glass-water interface.
It was developed to restrict the background fluorescence and increase the signal-to-noise ratio (s/n) in the resultant images. This is accomplished in TIRF by using the ability of light to create an evanescent wave (or field) at a very limited range within the sample beyond an interface of two substrates differing in refractive index. In practice, this involves imaging a specimen that is in direct contact with a glass slide or tissue chamber. If the angle of the light is greater than the critical angle, this refractive index mismatch will create a field/wave with properties that are identical in frequency to the light. In other words, a fluorescent molecule that would normally absorb light at 488nm can be 'excited' by the electromagnetic field created by a 488nm laser (or other monochromatic source) that is reflected off of the lower refractive index material. Since this field will decay in intensity exponentially with distance, the resultant fluorescence signal will occur in less than 100 nanometers of the surface. This effectively restricts the excitation to the sample and therefore reduces the z-axis signal which significantly increases the s/n ratio of the sample. Of course, this does mean that the molecules of interest must be within that limited range of the evanescent field.
TIRF has been used for some time and in a variety of fields. The initial work done with microscopy was with prisms and beam steering devices to bring the laser/light to the sample from above using an inverted microscope design. The recent developments of “through-the-lens” microscope systems (via high numerical aperture lenses) have made the application much more available to researchers who do not want to align their systems nor worry about lasers shooting around the room.
The "through-the-lens" approach to TIRF places an added burden on the optical filters since the reflected beam is collected by the objective lens and follows the beampath backwards. This means that the emission/blocking optics not only have to deal with huge amounts of excitation energy, but also high angle scatter and reflections from this light. A typical laser emission filter must block the excitation wavelengths to OD 6, but in TIRF this blocking must exceed OD 8 in most applications (note that many modern spectrometers will not even measure to OD 8 across the full visible spectrum). Our solution is to use proprietary blocking mechanisms and optics to achieve OD 8, even at the higher angles of these systems.
In addition to the added blocking requirements, the dichroic mirrors in these applications must be incredibly stable in reflected wavefront characteristics. Where a standard laser scanning confocal might require only a reflected wavefront distortion (RWD) of less than 2 waves per inch, TIRF applications require less than ½ wave distortion from the reflected surface. This reflected wavefront demand is after substrate coating as the coating process always exerts stresses on the fused silica substrates. This makes producing dichroics that meet these flatness requirements nearly impossible for some coating techniques and for some physical requirements of the substrates (i.e. very thin materials that are still fairly large in overall size).