







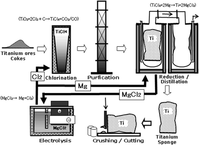
The Kroll process is a pyrometallurgical industrial process used to produce metallic titanium. It was invented by William J. Kroll in Luxembourg. After moving to the United States, Kroll further developed the method for the production of zirconium. The Kroll process replaced the Hunter process for almost all commercial production.
Process
Refined rutile (or ilmenite) from the ore is reduced with petroleum-derived coke in a fluidized bed reactor at 1000 °C. The mixture is then treated with chlorine gas, affording titanium tetrachloride TiCl4 and other volatile chlorides, which are subsequently separated by continuous fractional distillation. In a separate reactor, the TiCl4 is reduced by liquid magnesium or sodium (15–20% excess) at 800–850 °C in a stainless steel retort to ensure complete reduction:
2Mg(l) + TiCl4(g) ? 2MgCl2(l) + Ti(s) [T = 800–850 °C]
Complications result from partial reduction of the titanium to its lower chlorides TiCl2 and TiCl3. The MgCl2 can be further refined back to magnesium. The resulting porous metallic titanium sponge is purified by leaching or heated vacuum distillation. The sponge is jackhammered out, crushed, and pressed before it is melted in a consumable carbon electrode vacuum arc furnace. The melted ingot is allowed to solidify under vacuum. It is often remelted to remove inclusions and ensure uniformity. These melting steps add to the cost of the product. Titanium is about six times as expensive as stainless steel.